We provide support in carrying out quick and high-quality operations from introducing affiliated medical institutions to completing clinical trials.
We dispatch educated and trained CRCs to affiliated medical institutions to carry out secretariat work and all operations associated with clinical trials, such as dealing with subjects. We also engage proactively in raising public awareness for the implementing of clinical trials and establish infrastructure to ensure their smooth running.
Number of CRCs| The Japanese Society of Clinical Pharmacology and Therapeutics Board-certified CRCs | 9 |
| SMONA-certified CRCs | 25 |
| Internally-certified CRCs | 27 |
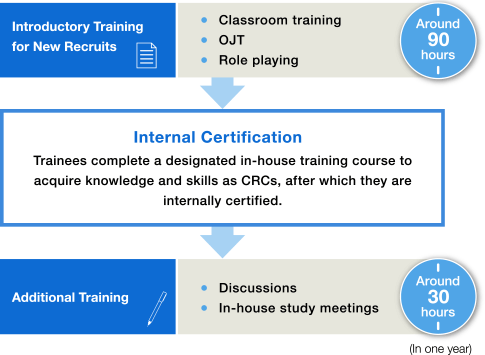
CRC Education System
[ External Training ]
● Conference on CRC and Clinical Trials
● The Japanese Society of Clinical Pharmacology and Therapeutics
● SMONA Seminar
● Participation in medical conferences and workshops, etc.
[ External Training ]

4th floor, FORECAST Ningyocho PLACE
3-4-14 Ningyocho, Nihonbashi,
Chuo Ward, Tokyo, 103-0013, JAPAN
Phone: 03-3662-0031
Fax: 03-3662-0033
Weekdays: 9:40 A.M. – 6:00 P.M.
Saturdays: 8:40 A.M. – 1:00 P.M.
Sundays and Public Holidays: Closed
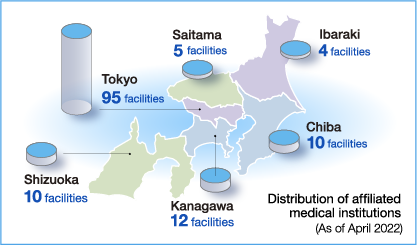
| Metropolitan area (Tokyo, Kanagawa, Saitama, Chiba) |
University hospital Hospitals Clinics |
1 5 116 |
| Ibaraki Prefecture (Mito, Hitachinaka, Naka County) |
Hospitals Clinics |
2 2 |
| Shizuoka Prefecture (Mishima, Shizuoka, Fukuroi, Fuji, Susono, Numazu, Fujieda) |
Clinics | 10 |




